
The primary issue with your intuition is merely understanding what cooling the substance down really does. As the cooling continues, the molecules move around less and less until they take on a definite shape, at which point it is considered a solid. All of these compounds are nonpolar and only have London dispersion. If the substance is cooled far enough, those forces are able to overcome the kinetic energy of the molecules and they condense into a liquid. This attractive force is called the London dispersion force in honor of German-born. But, as the molecules are cooled and slowed down ( this seems to suggest this is around 10m/s), London forces can cause more interactions between molecules. At those speeds, London forces don't really have much of an effect. While in the gaseous phase, the molecules have velocities of several hundred meters per second. I learned that what results in London fores between molecules is the 'cloud of electrons' that results in an instantaneous dipole and induced dipole, which eventually results into.

At this point, it's not that the dispersion forces because stronger, but rather that they become comparatively stronger. 'London (dispersion) forces are responsible for the fact that non-polar substances can be condensed to form liquids and sometimes solids at low temperatures'. So, the substance has been cooled down and molecules are moving more slowly. As mentioned in the comments on the question, this doesn't really affect the electron cloud. When we lower the temperature of a substance, we are decreasing the average molecular kinetic energy (they're slowing down). This is how I thought of it, can anyone suggest what's wrong with my way of thinking of it? Is it that what we relate to the strength of the London forces is how long the electrons cloud stay on one side rather than how often that happens?
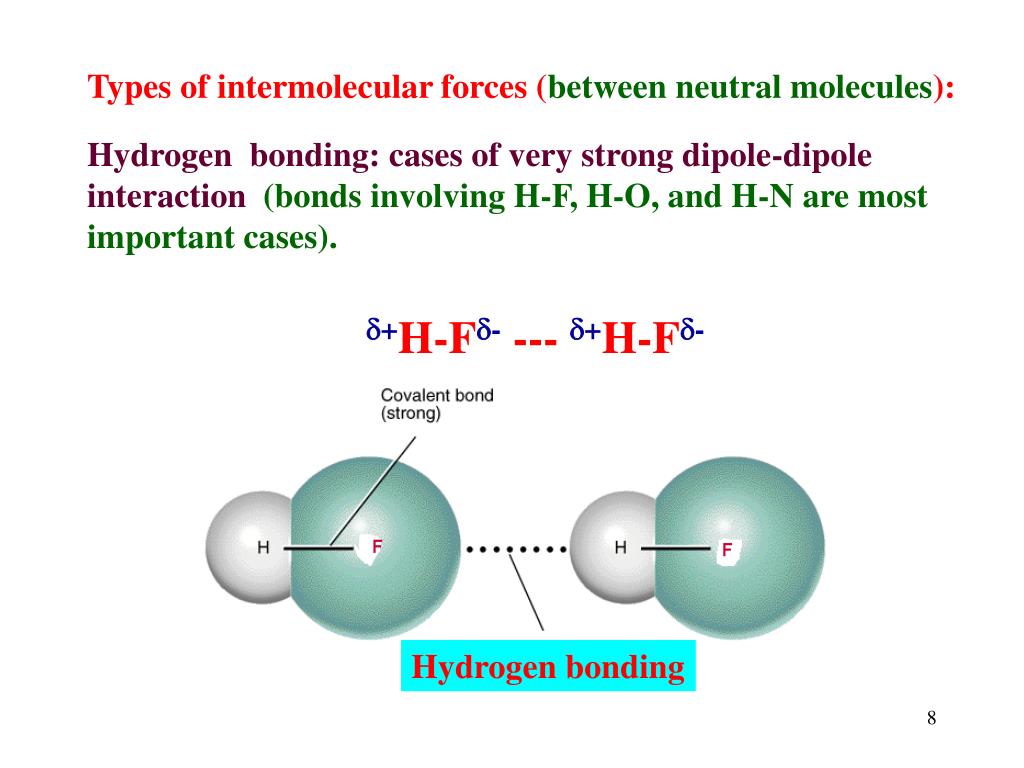
we decrease the frequency/probability of these "electron clouds" moving to one side of the molecule and forming instantaneous and induced dipole, which means that London forces should be weaker and not stronger to form a liquid or a solid. My question is: if we lower the temperature we subsequently decrease the kinetic energy of the electrons, i.e.

The electronegative difference between H and Cl makes a polar bond. The aforementioned statement suggests that as we lower the temperature we form a solid or a liquid meaning that the inter-molecular forces (London forces in this case)became stronger._ But it also has dipole-dipole forces because H-Cl has a polar bond. I learned that what results in London fores between molecules is the "cloud of electrons" that results in an instantaneous dipole and induced dipole, which eventually results into forming these weak London forces._ "London (dispersion) forces are responsible for the fact that non-polar substances can be condensed to form liquids and sometimes solids at low temperatures"._


 0 kommentar(er)
0 kommentar(er)
